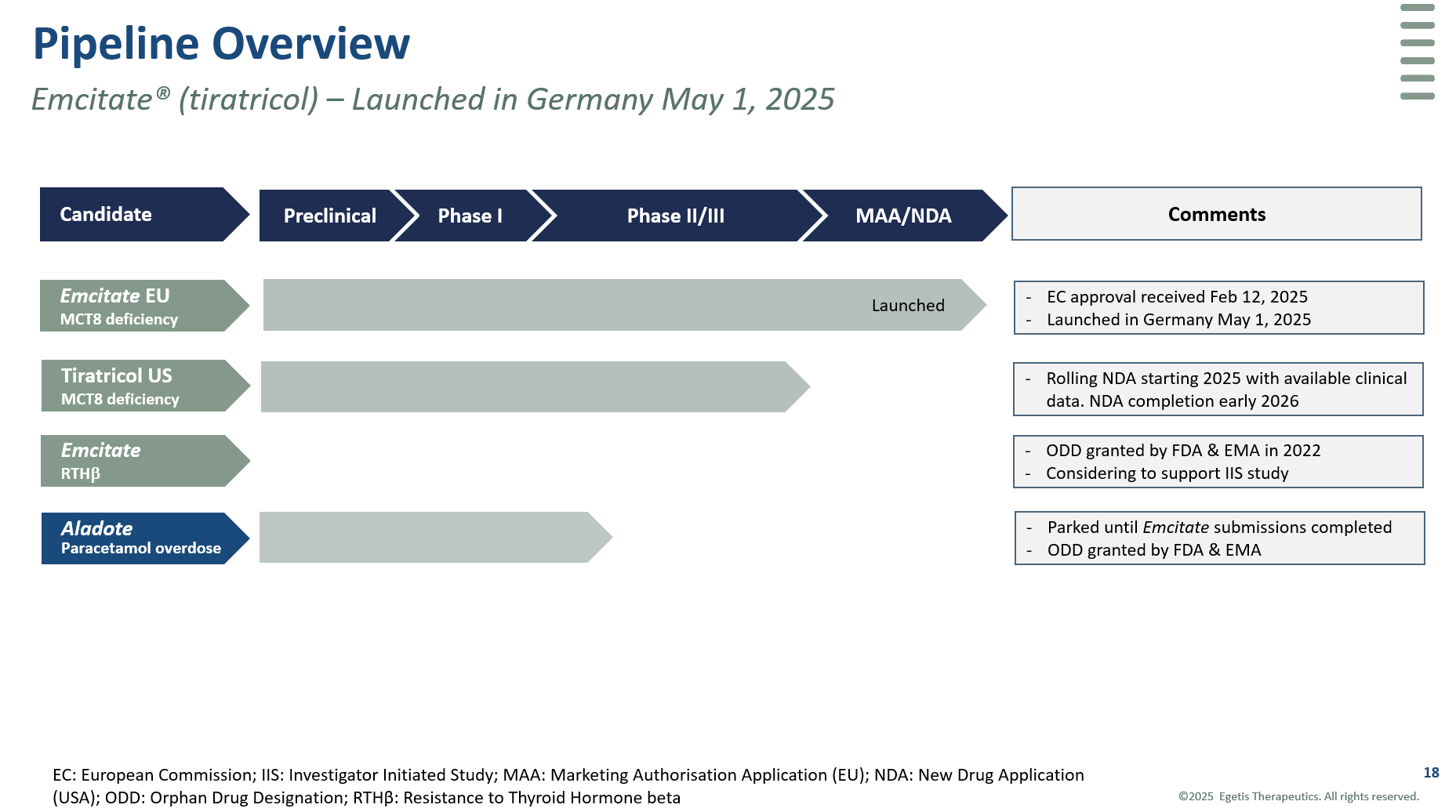
Pipeline
Emcitate® (tiratricol)
Emcitate is under development for the treatment of patients with MCT8 deficiency, a highly debilitating rare disease with no available treatment. In previous studies (Triac Trial I and a long-term real-life study) Emcitate has shown highly significant and clinically relevant results on serum thyroid hormone T3 levels and secondary clinical endpoints. As a result of regulatory interaction Egetis submitted a marketing authorisation application (MAA) for Emcitate to the European Medicines Agency (EMA) in October 2023. On December 12, 2024, Egetis received a positive CHMP opinion for Emcitate for the treatment of MCT8 deficiency.
In the US, after discussions with the FDA, Egetis is conducting a small randomized, placebo-controlled study in at least 16 evaluable patients to verify the results on T3 levels seen in previous clinical trials and publications.
Emcitate holds Orphan Drug Designation (ODD) for MCT8 deficiency and resistance to thyroid hormone type beta (RTH- β) in the US and the EU. Emcitate has been granted Rare Pediatric Disease Designation (RPD) which gives Egetis the opportunity to receive a Priority Review Voucher (PRV) in the US, after approval.
Aladote®
Aladote® a first-in-class drug candidate, is being developed to reduce the risk of acute liver injury associated with acetaminophen/paracetamol poisoning. A proof of principle study has been completed. Design of pivotal Phase IIb/III study for Aladote® finalized after completed interactions with FDA, EMA and MHRA. Aladote® has been granted Orphan Drug Designation in the US and EU.